 |
The Sullivan Lab, MCD Biology, UCSC |

| |||
Sullivan Lab Research II. Chromosome Biology: Entering and Exiting Metaphase with Damaged Chromosomes
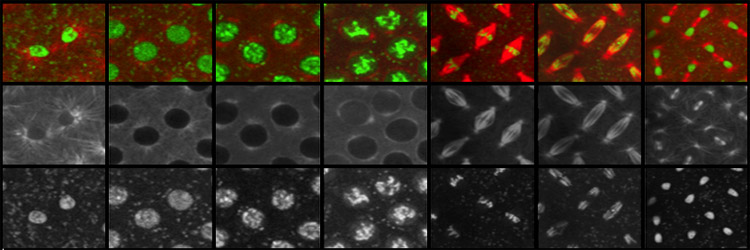
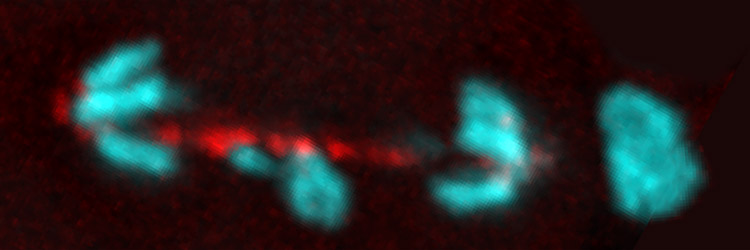
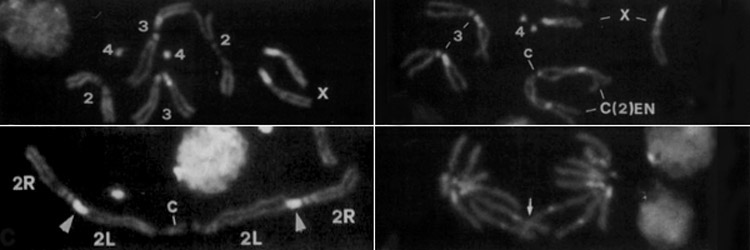
The eukaryotic cell has evolved a number of mechanisms to maintain genome integrity. In response to damaged or improperly replicated DNA, cell cycle checkpoints activate signaling pathways that delay cell cycle progression providing time to repair the lesions or eliminate the damaged cell. In spite of these safeguards, cells occasionally enter and exit metaphase with damaged chromosomes. Unrepaired double-strand breaks are particularly problematic because they produce chromosome fragment lacking centromere (acentrics) and thus are incapable of forming normal attachments with the mitotic spindle. Our lab focuses on the behavior of acentric and lagging chromosomes during anaphase and telophase. We are also interested in the adaptations of the mitotic apparatus to these wayward chromosomes. For example, late segregating acentric chromosomes lie in the path of the ingressing cytokinesis furrow. We have discovered these adaptations include dramatic changes in the size and shape of the cell and mitotic spindle. A particularly exciting recent discovery is that chromosome fragments lacking a centromere are able to efficiently segregate to the poles during the anaphase and become incorporated into daughter nuclei. This observation raises a number questions regarding the mechanisms of acentric segregation that we are currently addressing. In addition, because the segregation is delayed we find the anaphase cell must delay completion of nuclear envelope formation in order to provide time for inclusion of the acentric into the daughter nucleus. Representative Chromosome Biology Publications from the Sullivan lab Oliveira R A, Kotadia S , Mirkovic M, Bowlin K, Eichinger CS, Nasmyth K, Sullivan W(2014) Centromere-independent accumulation of cohesin at ectopic heterochromatin sites induces chromosome stretching during anaphase. PLOS Biology 12(10):e1001962. [PDF] Kotadia S*, Montembault E*, Sullivan W, Royou A. (2012) Cell elongation – an adaptive response clearing long chromatid arms from the cleavage plane. Journal of Cell Biology 199(5):745-53. [PDF] Fasulo B*,Koyama C*,Yu KR*,Homola EM, Hsieh TS, Campbell SD and Sullivan W (2011) Chk1 and Wee1 kinases coordinate DNA replication, chromosome condensation and anaphase entry. Molecular Biology of the Cell 23 (6): 1047-57. [PDF] Royou A, Gagou ME, Karess R , Sullivan W.(2010) BubR1- and Polo-Coated DNA Tethers Facilitate Poleward Segregation of Acentric Chromatids. Cell 140, 235–245. [PDF] For a complete list of publications related to chromosome biology, see our main Publications page. [Next] [Back] [Sullivan Research Main Page ] |
|||
|
|